molecular geometries
What Is Molecular Geometry?
Molecular geometry refers to the three-dimensional arrangement of atoms in a molecule. This spatial arrangement plays a crucial role in determining a molecule’s physical and chemical properties, such as polarity, reactivity, and biological function. Understanding molecular geometry is fundamental in fields like chemistry, biochemistry, and pharmacology.
Why Is Molecular Geometry Important?
The shape of molecules affects:
- Their polarity, which influences solubility and boiling points.
- Intermolecular forces such as hydrogen bonding and Van der Waals forces.
- The molecule’s reactivity and interaction with other molecules.
- Biological activity, including enzyme-substrate binding.
- Physical properties like color, magnetism, and phase of matter.
Studying molecular geometry allows scientists to predict and explain these behaviors, crucial in designing new materials, drugs, and understanding natural processes.
Theories Explaining Molecular Geometry
Valence Shell Electron Pair Repulsion (VSEPR) Theory
The VSEPR theory is one of the most widely used models to predict molecular shapes. It states that electron pairs (bonding and lone pairs) around a central atom repel each other and arrange themselves to minimize this repulsion, resulting in specific molecular geometries.
For example, in water (H₂O), two bonding pairs and two lone pairs create a bent geometry with a bond angle less than 109.5°.
Hybridization Theory
Hybridization involves mixing atomic orbitals to form hybrid orbitals that define the bonding and geometry of molecules. Common hybridizations include:
- sp Hybridization: Leads to linear geometry with 180° bond angles.
- sp² Hybridization: Results in trigonal planar geometry with 120° bond angles.
- sp³ Hybridization: Produces tetrahedral geometry with approximately 109.5° bond angles.
Molecular Orbital Theory
This theory explains bonding through molecular orbitals formed by the combination of atomic orbitals. It provides insights especially for molecules with delocalized electrons, explaining molecular stability and magnetic properties.
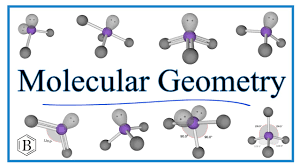
Common Molecular Geometries
Depending on the number of bonded atoms and lone pairs, molecules can adopt various shapes:
Linear
- Two bonded atoms, no lone pairs
- Bond angle: 180°
- Example: Carbon dioxide (CO₂)
Trigonal Planar
- Three bonded atoms, no lone pairs
- Bond angles: 120°
- Example: Boron trifluoride (BF₃)
Tetrahedral
- Four bonded atoms, no lone pairs
- Bond angles: 109.5°
- Example: Methane (CH₄)
Trigonal Pyramidal
- Three bonded atoms, one lone pair
- Bond angles: slightly less than 109.5°
- Example: Ammonia (NH₃)
Bent Geometry
- Two bonded atoms, one or two lone pairs
- Bond angles less than 109.5°
- Example: Water (H₂O)
Trigonal Bipyramidal
- Five bonded atoms, no lone pairs
- Bond angles: 90°, 120°, and 180°
- Example: Phosphorus pentachloride (PCl₅)
Octahedral
- Six bonded atoms, no lone pairs
- Bond angles: 90°
- Example: Sulfur hexafluoride (SF₆)
How Lone Pairs Affect Molecular Shape
Lone pairs occupy more space than bonding pairs, pushing bonded atoms closer together and reducing bond angles. This explains why molecules like NH₃ and H₂O deviate from idealized geometries like tetrahedral.
Hybridization and Its Influence on Geometry
Hybrid orbitals form depending on electron arrangements and dictate molecular geometry. For example, sp³ hybridization in methane creates a tetrahedral shape, while sp² hybridization in ethylene results in trigonal planar geometry.
Methods to Determine Molecular Geometry
Scientists use various experimental and computational methods to determine molecular shapes:
- X-ray Crystallography: Reveals exact 3D structures of crystalline molecules.
- Spectroscopic Techniques: Infrared and Raman spectroscopy give clues about bond vibrations linked to geometry.
- Computational Chemistry: Uses quantum mechanical calculations to predict molecular structures and properties.
Applications of Molecular Geometry
Understanding molecular geometry is crucial in:
- Drug Design: Optimizing molecular shapes to bind specific biological targets effectively.
- Materials Science: Designing polymers and catalysts with desired properties.
- Environmental Chemistry: Predicting pollutant behavior and interactions.
- Nanotechnology: Engineering molecules for precise functions and interactions.
Conclusion
Molecular geometry is a cornerstone concept in chemistry and related sciences. By combining theories like VSEPR, hybridization, and advanced computational techniques, scientists can predict, manipulate, and utilize molecular shapes for innovations in medicine, industry, and technology